Catalysis
By developing and understanding new catalytic processes we are making societally–important products, materials and chemicals more efficiently and with more precision.
Catalysis is the acceleration of a chemical reaction by a substance that is not consumed itself, and is crucially important to all areas of modern life. It is estimated that 85% of all products manufactured involve catalysis somewhere in their production chain, and such products have considerable impact in energy (petrochemicals), healthcare (pharmaceuticals), new–materials (polymers), transport (catalytic convertors) and environment (water, air quality, renewable and bio–produced materials). It is estimated that 90% of all chemical process are catalysed, and thus the economic impact of catalysis is huge, contributing 30–40% of global GDP. Imagine, for example, a world without ammonia (fertilisers), plastics, catalytic convertors or the ability to synthesise fine-chemicals for healthcare solutions.
The Chemistry Department at the University of Oxford has a critical mass of world–class researchers involved in catalysis. Their research areas are diverse across the theme, encompassing: small molecule and novel–materials synthesis, the development of routes to new pharmaceuticals and therapeutics, new energy vectors, new polymeric materials, efficient use of renewable resources, novel heterogeneous systems for chemicals essential for modern society, electrocatalysis, innovative healthcare solutions, chemical biology, theory and computation. A key aspect of catalysis research at Oxford is our drive to have a deep, fundamental understanding of the catalytic processes being developed and studied, partnered with a strong trajectory to deliver practical and useful solutions that have positive societal and industrial impact.
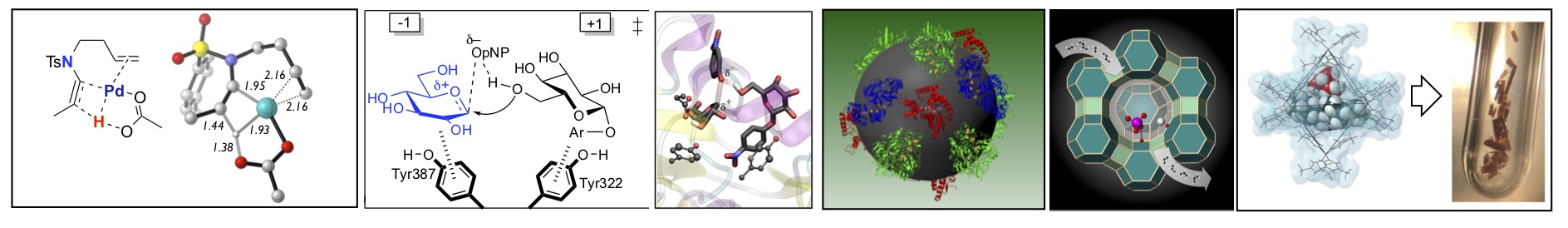
Illustration highlighting the many area of catalysis undertaken in the department: organometallic, organic, bio-organic, material and solid state.
Catalysis areas
Catalysis for the synthesis of fine chemicals
Catalysis is studied to explore the fundamental reactivity of carbon-based molecules and discover new ways to make or break bonds. Much of our work eyes unmet needs in the wider community and is often done in collaboration with leading chemical and pharmaceutical companies. As well as providing more efficient methods, catalysis can open new pathways to access molecules that could not easily be obtained otherwise. The Department has tremendous expertise in developing metal-, small molecule- and enzyme-mediated catalysis, application to target synthesis, and determining reaction mechanisms to facilitate development of new and further improved processes.
Catalysis for chemical biology and bioinorganic chemistry
Catalysis may allow chemically complex biological markers such as carbohydrates, proteins, nucleotides and secondary metabolites to be modified in order to understand or exploit biological function. Nature’s catalytic machinery can also be harnessed, immobilized, modified, and exploited to provide biotechnology solutions for fine chemical synthesis. Biologically derived methods also provide opportunities to explore single-molecule methods, reactions in unusual environments such as picolitre compartments and immobilized phases and allow mechanistic studies to understand the chemistry of catalysis which underpins important aspects of biology.
Catalysis for energy applications
Research in this sub–theme is focussed on providing solutions for new energy storage and generation solutions (for example fuel cell catalysis), and the cleaner, efficient and selective processing of key bulk chemicals used in transporting energy – such as petrochemicals.
Catalysis for the synthesis of new functional materials and molecules
Catalysis underpins the development of modern materials chemistry, and a number of groups are developing new catalytic methodologies for the production of new high–performance polymers, and the synthesis of new molecular materials that have specific, and tailored, electronic, structural and functional properties.
Catalysis using renewable and recyclable feedstocks
Catalysis is central to the efficient use of anthropomorphically derived CO2 and a number of groups are actively investigating the development of innovative catalysts that use CO2 as a chemical feedstock for the production of a wide variety of useful chemicals. The development of catalysts that offer fine control over the formation of polymers from renewable or recyclable bio-derived feedstocks that have applications as bio-renewable polymers, elastomers, coatings, matrices for tissue engineering or antimicrobial surfaces.
Catalysis also crosses many themes within Chemistry, including Advanced Functional Materials and Interfaces, Chemistry at the Interface of Biology and Medicine, Kinetics, Dynamics and Mechanism, Energy and Sustainable Chemistry, Synthesis and Theory and Modelling of Complex Systems.