- Researchers from Oxford Chemistry have discovered a way to asymmetrically add aromatic and heteroaromatic rings to racemic mixtures of non-cyclic, non-symmetric allyl systems.
- Their Suzuki–Miyaura-type reaction uses chelation to convert both enantiomers of non-symmetrical starting materials into a single enantiomer of product.
- Discovering novel ways to make chiral molecules enantioselectively is critical for drug discovery.
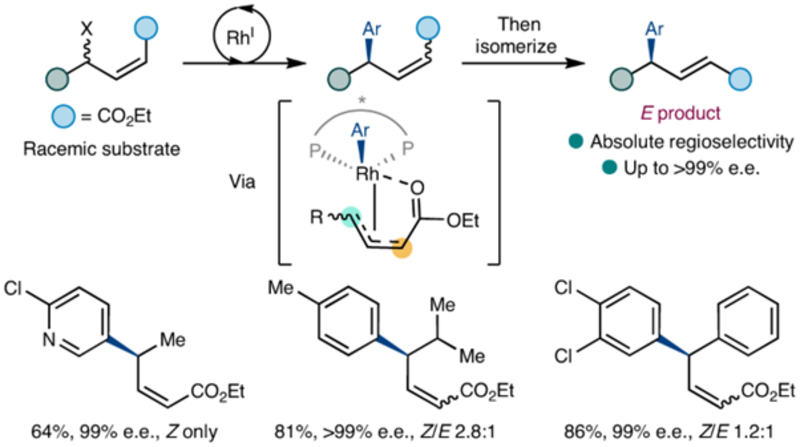
Asymmetric addition to a racemic starting material bearing an CO2Et group is used to generate a variety of functionalized products, and alkyl, branched and aromatic rings are tolerated at the centre where the stereogenic center is controlled.
In a study recently published in Nature Chemistry, Oxford Chemistry researchers describe a rhodium-catalysed Suzuki–Miyaura-type asymmetric addition reaction that shows almost complete chemo-, regio- and enantioselectivity when aromatic rings are added to racemic, non-symmetrical, acyclic systems. They speculate that a chelating group attached to the allyl system is key to allowing the many intermediates produced by oxidative addition to the allyl unit to converge onto a single product.
Suzuki-Miyaura cross-coupling reactions are powerful reactions for connecting two aryl rings, making them heavily used in drug discovery. There is evidence, however, that an overreliance on these reactions has skewed drug candidate libraries towards planar molecules. There is therefore a need to develop new Suzuki-Miyaura like reactions that are able to reliably produce small molecules with a wider variety of structures, and in particular single enantiomers of small molecules.
The Fletcher group has been developing asymmetric variations of Suzuki–Miyaura cross-couplings for years, where racemic mixtures were converted into single enantiomers of a product, but up until now these reactions were limited to using cyclic allyl cross-coupling partners that were symmetric about the allyl unit.
This method was used to synthesise biologically active and pharmacologically relevant molecules such as (S)-curcumene and the antidepressant sertraline (Zoloft®).
Lead author Dr Violeta Stojalnikova says:
There are vast possibilities that this new reaction unlocks, and we are excited to see all the creative ways medicinal chemists are going to use it.
“Chelation enables selectivity control in enantioconvergent Suzuki-Miyaura cross-couplings on acyclic allylic systems” is published in Nature Chemistry at https://www.nature.com/articles/s41557-023-01430-8.