Researchers at the University of Oxford, in collaboration with colleagues in Amsterdam and Okayama, have developed a powerful and general catalytic enantioselective method for building a class of molecules known as alkylidenecyclopropanes (ACPs). The team’s method is published today in Nature.
Critically, ACPs can be transformed into their cyclopropane counterparts. Cyclopropanes are small-ring carbocycles prized by chemists for their presence in biologically active compounds, including modern medicines and widely-used insecticides like permethrin. Until now, however, synthesising ACPs enantioselectively with high precision and purity has posed a significant challenge.
The new method uses a custom-designed class of superbase catalysts, called bifunctional iminophosphoranes (BIMPs), to guide a molecular rearrangement that relieves ring strain and precisely controls the shape (or stereochemistry) of the product. Remarkably, this catalytic process transforms simple-but-strained molecules made from commercially available starting materials into highly enantioenriched ACPs – molecules where all the atoms are arranged in a specific three-dimensional configuration – with excellent efficiency and under mild reaction conditions.
“This breakthrough shows how harnessing strain in small ring molecules can be a powerful strategy for building complex, chiral molecules with precision and purpose”, said Professor Darren Dixon, who co-led the study.
The ability to do this catalytically and selectively opens the door to new opportunities in medicinal chemistry, agrochemistry, and beyond.
In a major demonstration of the new method’s utility, the team applied it to the total synthesis of permethrin, a commercially important insecticide. They were able to access the biologically active stereoisomer in six steps and with 98% enantiomeric purity. The approach was also used to build the cores of other leading insecticides, all with high selectivity. Until now, producing such enantioenriched molecules required more wasteful techniques like chiral resolution, or stoichiometric use of chiral reagents.
Alongside the experimental work, cutting-edge computational modelling – led by Professor Trevor Hamlin of Vrije Universiteit Amsterdam – helped uncover how the BIMPs guide the reaction to deliver the desired stereochemical outcomes. These insights are expected to support further catalyst development and application to other difficult molecular transformations.
This study not only provides a new route to synthetically important targets but also highlights the power of combining rational catalyst design, experimental creativity, and theoretical understanding. It marks a significant contribution to the field of asymmetric synthesis and sets the stage for future developments in the construction of complex molecules for pharmaceuticals and crop protection.
Read more in Nature here.
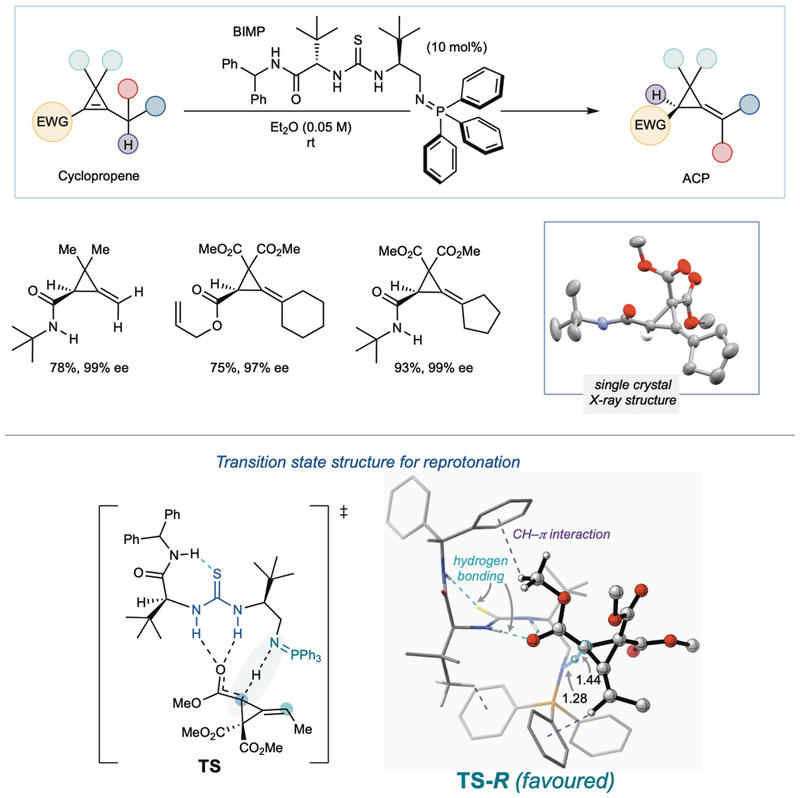
Scheme showing the researchers' newly developed method.