- Chemists have demonstrated the synthesis of a new family of molecular nanobelts that are larger and more electronically delocalized than any previous examples.
- A synthetic route to these porphyrin nanobelts was discovered unexpectedly and serendipitously, during the synthesis of linear ribbons, after many previous synthetic strategies had failed.
- These are the first molecules of this size (diameter >20 Å) to exhibit global aromatic ring currents in the neutral (uncharged) state, extending the Hückel 4n+2 rule to 120 π-electrons. The electronic wavefunctions of the nanobelts resemble that of a quantum ring, i.e. an electron delocalized around a circular path.
This study, published in Science, is part of a collaboration between the Universities of Oxford, Manchester, Nottingham and Ulm (Germany).
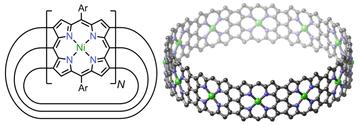
Left: Chemical structure of the belts (Ar is an aryl solubilising group). Right: Calculated structure of the 12-porphyrin nanobelt (N = 12; not showing solubilising groups). (From Science article.)
Molecular nanobelts have previously attracted interest as models for nanotubes. In this new study, triple-stranded porphyrin nanobelts have been synthesized consisting of 8 to 12 edge-fused porphyrin units with diameters of 21 to 32 Å. These nanobelts were synthesized by nickel-mediated coupling of dibromo porphyrins, to form singly linked nanorings, followed by oxidation with gold(III) chloride.
Scanning tunnelling microscopy of the singly linked rings on gold confirmed the circular structure and revealed that alternating porphyrin units sit parallel to the surface.
Experimental 1H NMR spectra, supported by computational simulations, showed that belts containing odd numbers of porphyrins, with circuits of 90 or 110 π-electrons, display global aromatic ring currents, whereas even-numbered belts, with 80, 100 or 120 π-electrons, are globally antiaromatic. These results are consistent with the predicted electronic structures of the belts, published before the experimental results (Vitek, et al. ACS Nano 2025, 19, 1405; https://doi.org/10.1021/acsnano.4c14100).
These nanobelts may find applications in molecular interferometers (Cheng, et al. Chem. Sci. 2025, 16, 4392; https://doi.org/10.1039/D4SC07992B).
You can read more about this study in Science.