For more than a century, chemists have known that molecules behave differently when pushed into excited states. As early as 1912, Giacomo Ciamician observed that photochemical reactions “often furnish surprises,” opening pathways unavailable to conventional chemistry. Today, visible-light energy-transfer (EnT) catalysis has transformed that vision into a practical synthetic strategy, allowing chemists to access triplet excited states under mild conditions. Yet despite its success, EnT catalysis remains constrained by a surprisingly old limitation: only a narrow set of functional groups, many identified over a hundred years ago, can be productively excited.
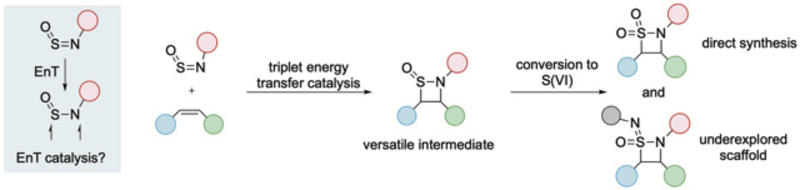
Sulfinylamines as a new group for energy-transfer catalysis, and their use in the synthesis 4-membered sulfur-containing heterocycles.
In a new study published in Science, researchers from the University of Oxford, led by Prof Michael Willis, and Pharmaron UK show how to break through this long-standing barrier. They demonstrate, for the first time, that sulfinylamines – a class of sulfur-containing compounds previously unexplored in energy-transfer catalysis – can be activated through visible-light excitation to drive synthetically powerful reactions. The result is an efficient and versatile route to four-membered cyclic sulfinamides, a family of molecules with direct relevance to medicinal chemistry that opens the door to unexplored chemical space.
The key advance lies in exploiting the excited-state reactivity of N-silyl sulfinylamines. Using commercially available photocatalysts and blue light, the team transfers energy to these reagents, generating triplet intermediates: high-energy molecular forms that contain unpaired electrons with aligned spins. These short-lived intermediates can then go on to engage alkenes in a [2+2] cycloaddition. This unconventional pathway bypasses the harsh conditions and highly polarized reagents required by previous approaches, instead accommodating a wide range of simple, readily available alkenes. The reactions are efficient, scalable, and compatible with batch and continuous-flow operation.
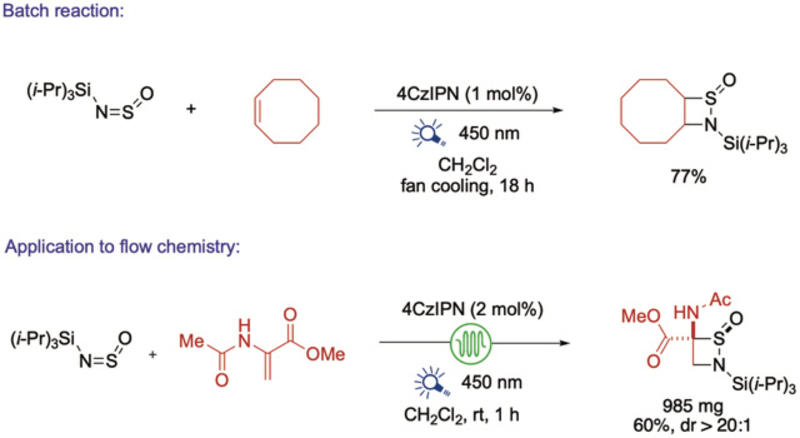
Batch and flow syntheses of 4-membered sulfinamides using visible light.
Why does this matter? Four-membered ring systems are notoriously difficult to make, yet they occupy privileged positions in drug discovery. β-Lactams, for example, underpin many of the world’s most important antibiotics. Their sulfur-based analogues, known as β-sultams, have shown promising biological activity but remain synthetically inaccessible. The cyclic sulfinamides reported here provide a direct and modular entry point to these targets. By simple oxidation and functionalization steps, the authors convert their products into β-sultams and, strikingly, into the first reported examples of four-membered cyclic sulfonimidamides, which are molecules that had previously eluded synthesis altogether.
Mechanistic experiments and quantum-chemical calculations reveal how the chemistry works. Energy transfer selectively excites the sulfinylamine, and the resulting triplet species reacts stepwise with alkenes through a diradical pathway, explaining both the observed selectivity and the failure of traditional thermal approaches. More broadly, the work establishes sulfinylamines as a new “excitable” functional group for EnT catalysis.
By expanding the palette of molecules that can participate in excited-state chemistry, this study shows how revisiting fundamental photochemical ideas can unlock entirely new transformations. The authors anticipate that sulfinylamine-derived triplet intermediates will enable many more reactions, helping chemists explore new regions of chemical space at a time when novel molecular scaffolds are urgently needed.
Read more in Science.