A team from Oxford Chemistry have synthesised a series of radical anions containing a rare four-atom nitrogen chain.
Nitrogen heavily disfavours chain formations, due to the disproportionally strong N≡N triple bond when compared to N–N single and double bonds. Radical anions of four-atom nitrogen chains have remained particularly elusive, only possible under extreme conditions such as high in the Earth’s atmosphere.
Now, the Mehta group at Oxford Chemistry have shown that a series of molecules containing {N4}•– can be reliably synthesised and studied. The five different molecules that the team made showed remarkable stability under ambient conditions, with one of the molecule’s lasting in the solid state for several weeks.
Reactivity studies showed that the chain can break down into N1 and N3 fragments, and can act as a source of nitrene radical anions.
Read more in Nature Chemistry.
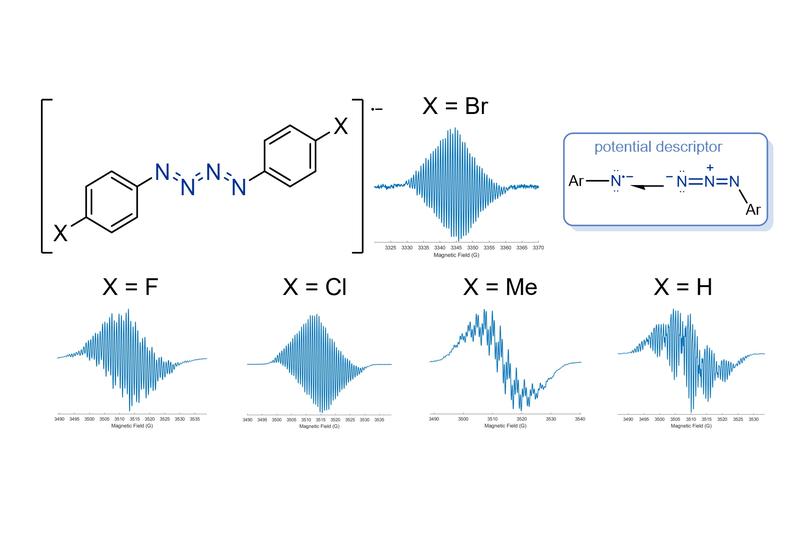
Isolated organo-{N4} radical anion chains and their EPR spectra under ambient conditions.