Nobel Prize in Chemistry for Oxford alumnus Richard Robson
Nobel Prize in Chemistry for Oxford alumnus Richard Robson
Richard Robson. Photo: Paul Burston/University of Melbourne
The Nobel Prize in Chemistry 2025 is today awarded to three chemists, including University of Oxford alumnus Professor Richard Robson FRS, for their work developing metal-organic frameworks.
Prof Robson receives the award alongside Susumu Kitagawa and Omar M Yaghi. The award recognises the creation of metal-organic frameworks, or MOFs – molecules with large spaces through which gases and other chemicals can flow.
MOFs have a diverse range of applications including harvesting water from desert air, capturing carbon dioxide, storing toxic gases and catalysing chemical reactions.
Heiner Linke, Chair of the Nobel Committee for Chemistry, said:
Metal-organic frameworks have enormous potential, bringing previously unforeseen opportunities for custom-made materials with new functions.
Professor Stephen Faulkner, Head of Oxford University's Department of Chemistry, said:
Richard Robson’s early work on coordination polymers and metal-organic frameworks combined original structural insights with practical innovation. He laid the groundwork for an entire field that is now fundamental to sustainable chemistry and molecular innovation. Many congratulations to all three recipients for their outstanding achievements.
Robson was born in 1937 in Glusburn, North Yorkshire. He matriculated at Brasenose College, Oxford in 1955, reading Chemistry and receiving a BA in 1959. He went on to study for a DPhil with John Barltrop in Oxford's Dyson Perrins Laboratory, which he completed in 1962, before moving to California for periods of postdoctoral research at the California Institute of Technology and Stanford University. In 1966 he moved to the University of Melbourne, where he remained for the duration of his career and is now an Honorary Professorial Fellow.
Professor Kylie Vincent, Associate Head of Department (People) in Oxford University's Department of Chemistry and an alumna of the University of Melbourne, said:
Richard Robson’s structural models and his gentle, thought-provoking teaching of solids have stayed with me from my undergraduate study at the University of Melbourne. When I visited Melbourne in recent years, Richard would ask fondly about his former colleagues from the Department of Chemistry in Oxford. I’m delighted to see this recognition for his foundational contributions to solid frameworks.
In 1974, Robson, then a lecturer in Melbourne, was tasked with creating large wooden models of crystalline structures for first-year lectures: sodium chloride, fluorite, and so on. By drilling holes at particular angles into wooden balls, he realised they became “pre-disposed to produce the structure that we were intending to produce”. He wondered whether molecules, if specially designed in a suitable shape, might similarly assemble themselves into ordered structures? This laid the foundation for the study of molecular self-assembly – although it was more than a decade before he finally started work in the lab to test this idea out.
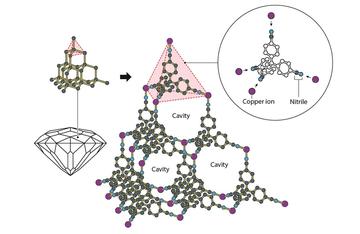
Richard Robson was inspired by the structure of diamond, in which every carbon atom is linked to four others in a pyramid-like shape. Rather than carbon, he used copper ions and a molecule with four arms, each with a nitrile at the end. This is a chemical compound that is attracted to copper ions. When the substances were combined, they formed an ordered and very spacious crystal. ©Johan Jarnestad/The Royal Swedish Academy of Sciences
In 1989 Robson showed that copper ions – known to prefer tetrahedral geometries – could crystallise with specially designed organic molecules that had four rod-like arms. The result was a spacious well-ordered crystal, much like the structure of diamond, but with large cavities between the molecules and metal ions (see figure).
Over the following decades Robson’s lab created a variety of molecular building block geometries. His work has since been built upon by Kitagawa, Yaghi, and countless other chemists.
Today, tens of thousands of different MOFs have been synthesised, with a variety of structures and properties. Some of these may contribute to solving some of the greatest chemical challenges of the future, including separating PFAS from water, breaking down traces of pharmaceuticals in the environment, and capturing carbon dioxide.




