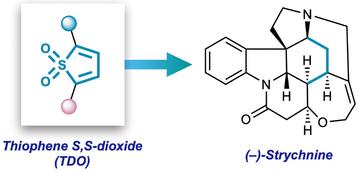
Thiophene S,S-dioxides used in this study, and iconic natural product strychnine.
Researchers from Oxford Chemistry have developed a new method to prepare eight members of an iconic family of naturally occurring molecules – the so-called 'Strychnos' alkaloids – by the shortest asymmetric synthetic routes devised to date.

Seeds of the Strychnos nux-vomica tree, from which the infamous poison strychnine is derived.
This family includes the infamous strychnine, a molecule notorious as a 19th century poison, of which Nobel prize-winning Oxford chemist Sir Robert Robinson said: 'for its molecular size, it is the most complex substance known.' The team were also able to synthesise its relative brucine, which had not succumbed to chemical synthesis in the 206 years since its discovery in 1819. This research, led by Prof Ed Anderson, DPhil student Kenny Park and Marie-Sklødowska Curie Fellow Jisook Park has been published today in Nature Chemistry.
Underpinning the project is the use of a doubly-oxidised heterocycle, called a 'thiophene S,S-dioxide' ('TDO' for short) as a key component in a Diels–Alder (4+2) cycloaddition reaction with an indole derived from the naturally occurring substance tryptamine. Upon heating, the TDO and indole undergo a cascade of chemical reactions driven by the loss of SO2, in which three new chemical bonds and two rings are created while forging four rings of the natural product framework.
Contemporary chemical synthesis of three-dimensional molecules brings an additional demand: to access just one enantiomer (i.e. a single mirror-image form) of the target. For this project, the team had to develop the first asymmetric cycloaddition reactions of TDOs, a new synthetic method in its own right.
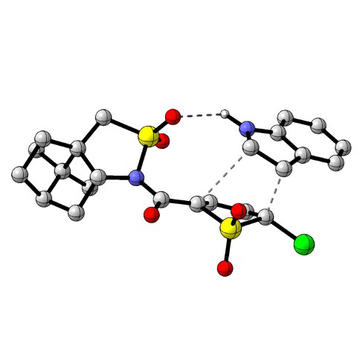
Transition state for the asymmetric cycloaddition of indole and thiophene dioxide.
Benefiting from the influence of a camphor-derived substituent on the TDO, the team achieved selectivities of over 20:1 for the desired product in the cycloaddition step, with many of these reactions proceeding at or below room temperature. Using this chemistry, the team were able to complete an enantioselective synthesis of the natural product akuammicine in just six steps, with a 20% yield over the entire sequence. They further completed syntheses of the flagship natural product strychnine, and the first synthesis of its relative brucine, among eight alkaloids in total.
A key aspect of the work was collaboration with Oxford computational chemist Prof Fernanda Duarte to understand the basis of the selectivity achieved in the asymmetric cycloaddition reaction of the TDO. Led by students Nils Frank and Hanwen Zhang, her group identified a series of factors including hydrogen bonding, structural distortion, and differing reaction mechanisms that rationalised the experimentally observed reaction outcomes. This benefited from important advances in computational chemistry techniques, such as the use of potentials optimised using a machine-learning approach for quantum mechanics calculations and molecular dynamics simulations.
Prof Anderson commented:
This was a truly exciting collaboration between experimental and computational research that establishes thiophene dioxides as new tools for efficient chemical synthesis. We're excited about the wider opportunities this chemistry offers, and are now looking at new applications of this under-explored heterocycle.
Read more in Nature Chemistry.
Images: Strychnine tree/seeds via Wikimiedia Commons (Lalithamba/H. Zell).