A team from Professor Ed Anderson's group, working in collaboration with the Duarte and Schofield groups in Oxford Chemistry, the Brennan group in the Nuffield Department of Medicine, and industrial partner AbbVie, have developed a new hydrocarbon mimic of meta-substituted benzene rings that can be used as an arene replacement in drug design.
Published in Nature, the team discovered that this hydrocarbon mimic, known as a 'bicycloheptane', can be accessed using ring-opening reactions of the strained molecule '[3.1.1]propellane'. Rigid small ring hydrocarbons have become popular in drug design as they can improve the biophysical properties of drug candidates compared to 'traditional' molecules containing benzene rings. However, while mimics for para-substituted benzenes are well-established, a meta-arene surrogate was lacking from the arsenal of the medicinal chemist. This is the challenge that this research addressed.
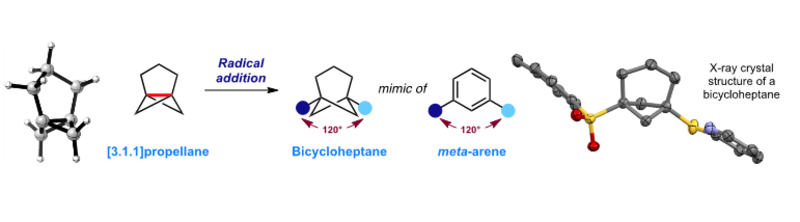
The team's first objective was to develop a scalable synthesis of [3.1.1]propellane, which although known for over 40 years, had hardly been explored before this study. The synthetic route designed now enables the preparation of multigram quantities of [3.1.1]propellane, which the team found reacts readily with a wide range of free radicals to form the bicycloheptane product. Using X-ray crystallography and theoretical calculations, they showed that the bicycloheptane is a near-perfect mimic of the geometry of a meta-substituted benzene ring.
Nils Frank, a Masters student in Prof. Anderson's group and lead author of the study, says:
This project taught us how closely fundamental and applied research can sometimes lie. It is really exciting to see how theoretical interest in the reactivity of higher propellanes has led to a collaborative project using this exotic molecule to form meta-substituted benzene bioisosteres.
The team were also able to show that bicycloheptane analogues of existing drug molecules can be readily synthesised, such as the anti-cancer agent Sonidegib. They compared the pharmacological properties of these analogues with the authentic drug compound, and found that the bicycloheptane is more resistant to metabolism by liver enzymes than the parent drug, and also more easily transported across a membrane, which is important for uptake of drug molecules in the body.
Prof. Anderson says:
We are very excited to develop this first true mimic of meta-substituted benzene rings, which are commonplace in many drug molecules. The availability of structures such as this, which can improve the properties and even bioactivities of drug candidates, is a really important goal for the medicinal chemistry community. We're looking forward to seeing it in use!