Biomimetic oscillations in a self-replicating chemical system
Biomimetic oscillations in a self-replicating chemical system
A team from Stephen Fletcher’s research group have shown how a chemical system of self-assembling, self-replicating molecules can exhibit oscillations in time. In a paper recently published in Nature Chemistry the researchers showed how simple cell-like structures can emerge, decay, and then repeatedly arise again in a network of relatively simple chemicals.
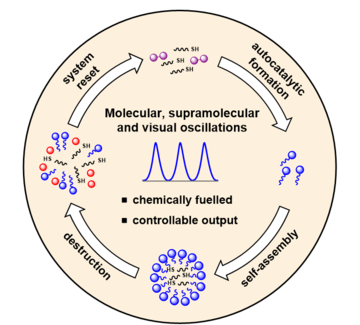
Figure 1: Oscillating reaction cycle.
In the study they analysed the reaction between a hydrophilic disulfide (A, purple molecule in Fig. 1) and hydrophobic thiol (B, black) to form a molecule (C, blue) that can self-assemble into micelles. By carefully controlling the reaction parameters, for example stirring rate, they were able to observe sustained oscillations in the number of micelles formed over time.
Micelles are spherical supramolecular structures held together by weak intermolecular forces. As well as being important in many biological processes they are also used in drug delivery and in chemical syntheses, because the interior of the spherical shell can hold molecules like a mini reaction vessel and lead to enhanced yields.
All known forms of life operate on length scales beyond the molecular, with supramolecular structures like micelles, vesicles and protein host-guest complexes critical to countless important biological functions. Designing supramolecular replicators capable of sustained oscillations would be a big step towards creating systems with this lifelike complexity, and would give insight into how complex systems could emerge and evolve.
Because the diameter of the micelles in their oscillating system is on the order of nanometres, the team could image their growth and decay in the solution by performing super-resolution scattering microscopy (iSCAT), taking snapshots at regular intervals and counting the number of particles. On a timescale of hours, the number of micelles rose and fell reliably and repeatedly, a result that was also demonstrated using liquid chromatography on samples from the reaction mixture.
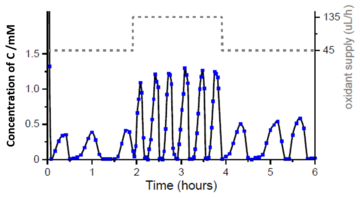
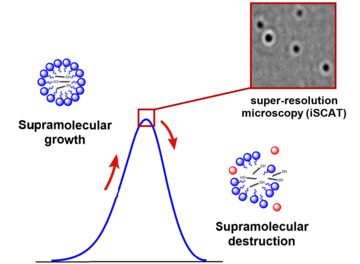
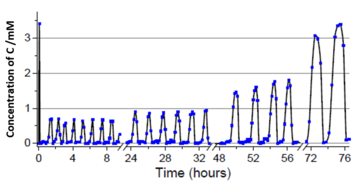
Figure 2: The concentration of C oscillates in time on the scale of hours or days, responds to the amount of oxidant supplied (H2O2) and shows repeated micelle growth and decay, as visualised by chromatographic analysis and scattering microscopy (iSCAT).
The proposed sequential processes that lead to oscillations in the amount of micelles is shown in Fig. 1. Both the formation and destruction of micelles requires B, which is taken up into the centre of the spherical structure. Importantly, only the micelle formation requires A. When both A and B are present the production of C is favourable, whereas when A is depleted the micelles release B to cause the breakdown of C and therefore the micelles themselves. This process represents one oscillation, or one loop around the circle in Fig. 1, after which the system resets.
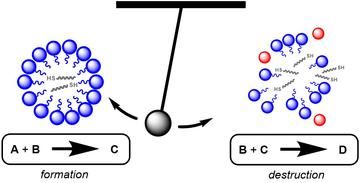
Figure 3: The reaction swings over time between micelle formation and destruction processes.
The balance between the catalysed formation and destruction of component C is critical for the observation of oscillations – the reaction swings back and forth like a pendulum, oscillating between enhanced production of micelles and enhanced destruction (see Fig. 3). All oscillating chemical reactions require some kind of autocatalytic process that can lead to chemical feedback, whereby large increases in the concentration of one component overshoot and then lead to large decreases.
DPhil student Michael Howlett, of the Synthesis for Biology and Medicine CDT and joint lead author alongside Anthonius Engwerda, describes the long experiments the team ran in order to track the progress of the oscillations over a day:
When first discovered, the oscillations were very chaotic and we spent a lot of time taming them, but with the two of us working together to monitor the reactions in high resolution, we could even track the oscillations over multiple days and are really proud of how far it has come!
The dynamic behaviour of this oscillating system across different length scales may have relevance in drug delivery, signal transduction, and in the design of functional nanoscale machines. Going forwards the aim is to further control these oscillations not only in time but in space – because the micelles can take up and release chemicals, it is hoped that in the future it may even be possible to design systems that could directionally transport chemicals.




