Around half of all chemotherapy treatments for cancer involve platinum compounds, such as the well-known cisplatin, despite the often-serious side effects of these highly reactive complexes. These platinum-based treatments form crosslinks with DNA and proteins in cancer cells, ultimately resulting in cell death.
Meanwhile, lanthanide-containing compounds are widely used in magnetic resonance imaging (MRI) for disease diagnosis. In MRI imaging a strong magnetic field interacts with the protons in water throughout the body, with the level of contrast in the MRI image strongly depending on the rate at which the nuclei’s magnetization returns to normal. This relaxation rate can be affected by the magnetic properties of surrounding chemical compounds, and intravenously administered lanthanide metal complexes are commonly used as contrast agents, increasing the contrast in MRI imaging of blood vessels and helping to diagnose brain tumours where the blood–brain barrier has degraded.
In a joint study between the Farrer, Faulkner, and Baldwin research groups, a range of lanthanide–platinum compounds have been synthesised that were shown to permeate and accumulate inside cancer cells. The build-up of these compounds inside the cells was measured using magnetic resonance, and it was shown that a version of the compound without a platinum complex was not toxic to the cells, whereas the platinum-containing compound was. Depending on the oxidation state and bound ligands of the platinum complex, the initially low toxicity of some of the compounds increased over time as the oxidation state of the platinum complex was reduced from Pt(IV) to Pt(II) inside the cell – this activation in the cell is an attractive feature for targeted chemotherapy that will hopefully reduce unwanted damage to non-cancerous cells.
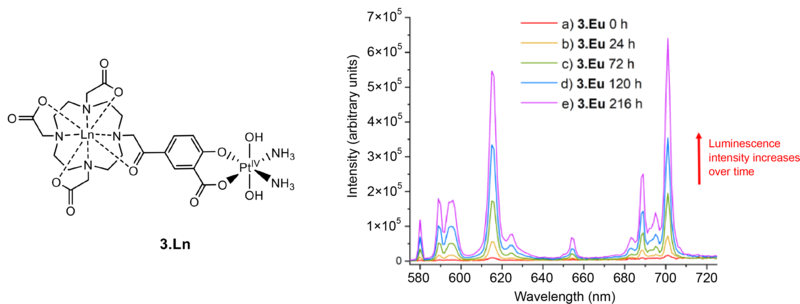
An example of one of the lanthanide–platinum complexes synthesised in the study, labelled 3.Ln. The luminescence intensity of the complex increases after the addition of ascorbic acid, shown by the coloured lines over a period of nine days (216 hours) for the case when the lanthanide chosen is europium (3.Eu). The ascorbic acid reduces the platinum from Pt(IV) to Pt(II) in an example of “switch-on” luminescence.
The results of the study, published in the journal Dalton Transactions, suggest that the novel gadolinium- and europium-containing compounds have significant potential for development, both as an imaging probe that can permeate inside cancer cells, and as a method of studying real-time accumulation of platinum prodrugs (compounds with little or no pharmacological activity that are chemically transformed into an active form in the body).
Dr Nicky Farrer says that:
Switch-on lanthanide luminescence following the reduction of a platinum(IV) anti-cancer prodrug is really exciting. It will enable us to visualise the reduction of the compounds at a sub-cellular level, and help us to understand more about their mechanisms of action.
In the long term, the hope is that this may lead to the development of novel targeted anti-cancer drugs.
Reference:
Yao, K. et al., Cell-permeable lanthanide–platinum(IV) anti-cancer prodrugs, Dalton Transactions 50 8761 (2021)
Banner photo of human cancer cells by National Cancer Institute on Unsplash.