Researchers from Oxford Chemistry have developed a new catalytic strategy to prepare enantioenriched phosphorus(V) centres.
In a study published in Nature Chemistry this week, the team describes how they were able to synthesise classes of molecule that have applications in medicine, agrochemistry, and catalysis. In contrast to existing methods, their new adaptable technique provides access to not just one but many classes of biologically relevant phosphorus compounds, and with high stereocontrol.
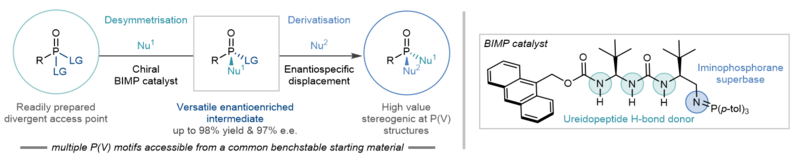
Summary of the strategy developed by the Oxford team, showing the desymmetrisation and derivatisation steps that lead to a wide range of enantioenriched stereogenic P(V) structures.
The new method has two phases: firstly, a prochiral nitrophenol-derived phosphonate ester undergoes a catalytic reaction with a nucleophile of choice to produce an enantioenriched intermediate in high yield and high enantiomeric excess. In the second step another nucleophilic substitution takes place enantiospecifically, whereby a variety of N-, O-, and S- based nucleophiles can be used to access a wide range of enantioenriched stereogenic P(V) molecules.
The team demonstrated that a wide variety of high value P(V) compounds can be obtained with very high enantiospecificity, in some cases 100%. To gain insight into the origin of the high enantioselectivity observed in the desymmetrisation step, Dr Ken Yamazaki of Okayama University led computational studies that revealed the rate- and enantio-determining step of the reaction is the addition of the nucleophile to the P(V) electrophile in the first desymmetrisation step (see figure).
The catalysts and computational work developed in this study represents a leap forward to more general, efficient, and modular methods to synthesise these important P(V)-containing molecules.
Read more about the study in Nature Chemistry.