Congratulations to a team from the Department of Chemistry who have won second prize in the 10th SCI-RSC National Retrosynthesis Competition, held in March.
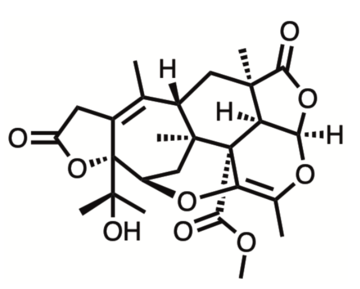
Talaromynoid A (see Journal of Natural Products 2021 84 (10), 2727-2737 DOI: 10.1021/acs.jnatprod.1c00681)
Every year, a novel complex natural product is chosen from the scientific literature by the SCI's Young Chemists’ Panel, SCI's Fine Chemicals Group and RSC’s Heterocyclic and Synthesis Group. This year, the molecule was the complex monoterpenoid Talaromynoid A, first isolated in 2021 – there is currently no known synthetic route towards its preparation.
Chemists from both industry and academia assembled teams to propose a retrosynthesis and forward synthesis towards the natural product, providing a platform for them to demonstrate their synthetic and problem-solving skills. The panel then selected 10 teams to present their proposed route, followed by an intensive grilling by a team of judges and an audience of chemists across the country at Burlington House in London, home of the Royal Society of Chemistry.
The teams are judged based on which route balances elegance, novelty, and scope for success in the lab. The day was filled with an awe-inspiring and diverse range of synthetic methodologies, highlighting the beauty and elegance of organic synthesis.
The team from Oxford, named “SynCity”, was made up of Yasmine Biddick (2nd year DPhil student, Ed Anderson), Aidan Kerckhoffs (final year DPhil student, Matthew Langton) and Pavle Kravljanac (3rd year DPhil student, Ed Anderson). They came second to a team from pharmaceutical company Pfizer.
The Oxford team proposed to prepare the natural product in a projected 21 longest linear step sequence, which featured a dianionic Ireland-Claisen rearrangement to generate the challenging contiguous quaternary centres in the molecule. They also employed a late-stage photoredox cyclization to close up the complex ring system.