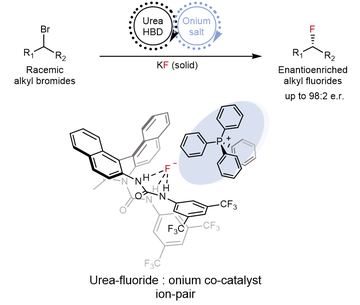
Enantioconvergent synthesis of alkyl fluorides with KF under synergistic phase transfer catalysis via the formation of a urea-fluoride-onium ion-pair (pictured)
In a study published today in Nature Catalysis, chemists from the Gouverneur group at the University of Oxford report a new way to use potassium fluoride (KF) for nucleophilic fluorination.
Their novel method, developed in collaboration with the Lloyd-Jones group (University of Edinburgh) and the Paton group (Colorado State University), employs a synergistic phase transfer catalytic manifold for the enantioconvergent synthesis of alkyl fluorides.
In 2018, Gouverneur and co-workers reported that chiral urea catalysts bring otherwise insoluble alkali metal fluoride salts into solution through hydrogen bonds, forming enantioenriched organofluorine compounds. This novel catalytic concept, termed hydrogen bonding phase transfer catalysis (HBPTC), relied on the formation of ion pairs between urea fluoride species and onium electrophiles, such as episulfonium, aziridinium or azetidinium ions for enantioselective fluoride delivery.
The group has now formulated a solution for a significantly more challenging process: the fluorination of neutral electrophiles with KF. This was achieved by introducing an onium halide co-catalyst to enable fluoride solubilisation as a ternary urea-fluoride-onium complex, thereby fulfilling the ion pairing requirement previously met by cationic electrophiles. These conditions enabled the enantioconvergent transformation of racemic starting materials into enantiopure products, overcoming the high waste associated with kinetic resolution and chiral separation processes which are commonly used in industry.
The Gouverneur lab now aims to further explore this concept, investigating the potential of synergizing hydrogen bonding phase transfer catalysis with other catalytic manifolds. Such an approach could enable the synthesis of a wider range of fluorinated compounds using cost-effective and earth-abundant fluoride salts.