George Fleet

Professor George Fleet
Emeritus Professor
Professor George Fleet is an Emeritus Fellow of St John’s College and was Professor of Chemistry in the Department from 1989 to 1997.
Undergraduate at Magdalene College Cambridge UK, Ph D with Ian Fleming at Cambridge on heterocyclic analogues of benzyne. MRC Post doctoral fellow at Oxford with Jeremy Knowles [Dean of Harvard] and Rodney Porter [Nobel prize winner] on the Photoaffinity labeling of antibodies by nitrenes derived from azides. Post doctoral work with E J Corey [Nobel prize winner] at Harvard on prostaglandin synthesis and chromium(VI) oxidation. At Oxford since. Visiting Professor at National Engineering Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang. China from 2010. Emeritus research professor St John’s College Oxford 2011. From 2013-2015 Emeritus Leverhulme Research Fellow. Visiting Professor at International Institute of Rare Sugar Research and Education, University of Kagawa, Kagawa, Japan from 2016.
Research interests: Synthesis and investigations of rare and expensive sugars with only acetone as protecting groups; adding high value cheaply with industrial scale production. Collaborative power of chemistry and biotechnology. Isolation and synthesis of missed bioactive water-soluble low molecular weight nitrogen compounds from plants which are used as sources of food. Pharmacological chaperones: iminosugars as small molecules to assist correction of protein mis-folding diseases including cystic fibrosis and lysosomal storage diseases – Tay-Sachs, Sandhoff, Schindler-Kanzaki, Fabry. Synthesis of iminosugars of all stereoisomers from cheap carbohydrates by convergent syntheses for the systematic investigation of their properties as glycosidase inhibitors; novel anti-cancer strategies. Development of general syntheses of highly functionalized azetidines from sugars. Azetidine carboxylic acids as novel peptidomimetics. Sugar amino acids as scaffolds for the study of foldamers.
Publications and patents: A full list of publications and patents, which runs from the most recent backwards over 7 !! decades, is given below. A reliable assessment of published work for chemists is the Hirsch-index. The group H-index is 72 - H index 24 since 2016; more than 120 papers with >50 citations; total >21.7K citations.
Green environmentally friendly synthesis of novel sugars – imino sugar mimetics that may cure cancer by several different mechanisms – novel antiviral compounds – probiotic and prebiotic foods for the future – treatment of obesity - functionalized amino acid scaffolds – new high value added intermediates by green methods – some nucleosides.
Green syntheses of 2-C-methyl nucleosides – to cure hepatitis C?
At present there is no satisfactory treatment for hepatitis C; there are 150 million people in the world who are chronically infected. There are some promising compounds making progress such as the Novartis-Idenix 3-O-valine ester of 2-C-methyl cytidine 1. Compounds following up behind are the Pharmasett 2-C-methyl fluoride Roche 2 and the Roche 2-C-methyl azide 3. The synthesis of such compounds needs easy access to a sugar in which the carbon chain is branched is key – and made by green technology without protection or environmentally unfriendly metals.
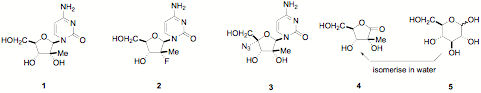
No branched sugars have ever been commercially available before. The sugar that is needed for many such syntheses is 2-C-methyl-D-ribonolactone 4 – we have worked with Novartis-Idenix so that 4 can be made in water on an industrial scale - with the power of biotechnology this will open up a diversity of monosaccharides that have no precedent. D-Glucose can now be converted in water in yields of well above 25% to 4. We also work on the complete synthesis of such branched nucleosides that completely avoid the environmental problems of protection inherent in present approaches to such drugs; a green way to antiviral nucleosides.
Green syntheses of new branched chirons
A general synthesis of carbohydrates bearing methyl branches at C-2 is shown below. Some are easier to do than others – all are likely to be useful high value intermediates.
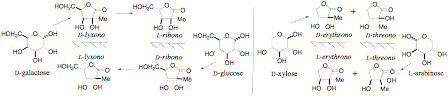
The main research areas has been the synthesis of novel carbohydrates with branched carbon chains – we believe that this will lead to new classes of bioactive compounds which will provide novel structures with important anti cancer and antiviral properties. We also have green approaches to C-3 and C-4 alkyl substituted sugars. We are using the products to make complex enantiomerically pure targets with functional groups at quaternary positions.
Rare and novel carbohydrates
Although no new sugars were newly available for 50 years, the drive of alternative food stuffs – which look like sugar, taste like sugar and cook like sugar but have few calories and many beneficial side effects – has led to an explosion in the number of potential new carbohydrate building blocks. One example of a rare sugar is D-tagatose which occurs at around 8% in Rocquefort cheese. It is made chemically from whey; the price of D-tagatose has dropped from $10,000 per kilogram to $5 per kilogram. However the conceptual biotechnological advance of Izumoring means that any monosaccharide would be available in water under green and environmentally conditions. The lack of glycaemic response and the stimulation of insulin production is likely for these new monosaccharides to improve the live style of diabetics as well as reducing obesity; many other positive biological properties of these novel sugars are being found that may be useful in the treatment of artherscelorosis, cancer, diabetes, and heart diseases.
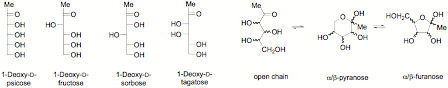
We did collaborative work with Professor Izumori; we made for the first time in significant amount all the diastereomers of the 1-deoxy-ketoses in syntheses. We have looked at the structure in the solid state by X-ray crystallography [Fig 1 and Fig 2] and in solution by NMR.
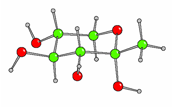
Fig. 1. Cystal structure of 1-deoxy-a-D-sorbopyranose
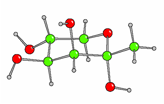
Fig 2. Cystal structure of 1-deoxy-a-D-tagatopyranose
Second generation imino sugars – antiviral agents – novel immunomodulators
There are many targets in imino sugars with specific biological targets. One recent example is the design of a synthesis of substantial quantities [around 50 g] of casuarine 1D; the key properties of casuarine, with 6 adjacent chiral centers, are discussed in the patent literature.
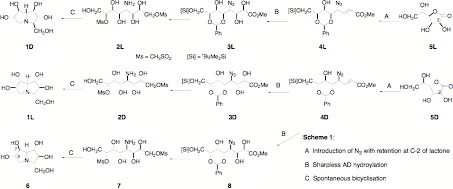
The synthesis of casuarine starts from L-gulonolactone 5L in which the introduction of azide with retention of configuration at C-2 and chain elongation gives 4L; hydroxylation to 3L allows the development of the completely unprotected amino dimesylate with 5 unprotected hydroxyl groups. This cleanly cyclises to the required target. The mirror image 1L has been made from D-gulonolactone 5D, along with another diastereomer 6.
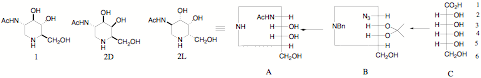
The GlcNAc analogue 1 has interesting properties We have a project to make both enantiomers GalNAc analogue 2D and 2L for potential anticancer properties. We are making it from the allonic acid enantiomers C and we will update this page with how we get on and the biological properties.
Doubly and multiply branched sugars
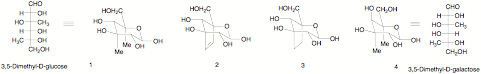
The major interest in developing the large scale preparation of hitherto unavailable sugars has been as nutrichemicals which have most of the taste and cooking properties of the common carbohydrates but few of the calories. It has also become clear that the monosaccharides themselves have other useful chemotherapeutic properties; although it is likely that any carbohydrate needed on a large scale would be produced by biotechnological procedure, the initial preparation is likely to be chemical. The aim of this project was to make sugars with two or more carbon branches. There are very few doubly carbon branched sugars; the rare examples are in nucleoside chemistry in which they are only reported in a protected form suitable for making nucloside analogues. There has been no study of the structure of such doubly branched unprotected monsaccharides in solution. It is likely that studies on the change in conformation or their water solubility and lipophilicity – will they still be soluble in water with 5 C-methyl groups. We predict that 3,5-dimethylglucose 1 which in the chair form would have the axial methyl groups will have activity against glycogen phosphorylase (GP) as well as the rigid form 2 and its reduction product 3. We would also aim to prepare 3,5-dimethyl-D-galactose. Both enantiomers of the sugars would be prepared.
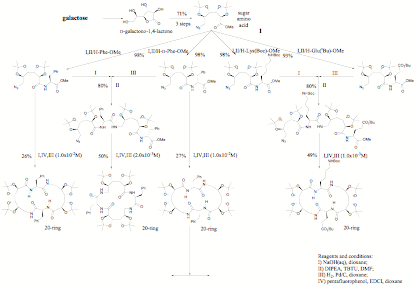
Large lactams: synthesis, do they bind can you attach them to solid phases?
The sugar amino acid building block 1 derived practically from galactose can be used to make large rings up to 70 membered; this was first made in the hope of making hydroxylated nylon polymers but they cyclised too easily. From CD studies it is clear than such cyclic materials have real potential to bind substrates; the original work has no way of having a side chain and the structure is too symmetrical for any NMR studies. The scheme below does.
Synthesis of carbohydrate mimics and sugar amino acids with one or more quaternary carbon:
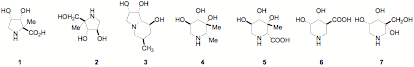
Although there has been much interest in hydroxylated prolines, analogues with a quaternary centre such as 1 have been difficult to access; a series of now available sugar lactones with a branched carbon chain have made access to such prolines practical, The C-methyl pyrrolidine 2 is an analogue of the naturally occurring glucoside inhibitor DAB which is in trial for the treatment of diabetes. We have report that the methyl substitution in L-swainsonine 3 is an order of magnitude more powerful inhibitor of nariginase than is the unbranched indolizidine; in contrast the additional methyl group at C-4 in the DFJ analogue 4 causes a loss of potency in fucosidase inhibition. The branched sugar lactones also give easy access to piperidine amino acids such as the pipecolic acid 5 and the nipecotic acid 6 as well as hydroxyisofagomine analogues 7. The consequence of methyl substitution in bioactive materials is being investigated.




