Our research interests lie in synthetic organic chemistry, and the contribution that this science can make to the fields of medicine and natural products synthesis. We concentrate on developing new catalytic reactions and methodologies for synthetic organic chemistry and then employing our chemistry to make biologically important natural products. Our work is directly relevant to the pharmaceutical industry (the research group is supported by many multi-national companies) and we love working on topics such as hydrogen borrowing, metathesis and dearomatisation reactions. The facilities available for research in our labs in the CRL are second to none. The research group consists of three Part IIs, ten DPhil students and four postdoctoral assistants. We meet at least twice a week to discuss advances in the laboratory and to hold problem sessions which highlight recent advances in the literature.
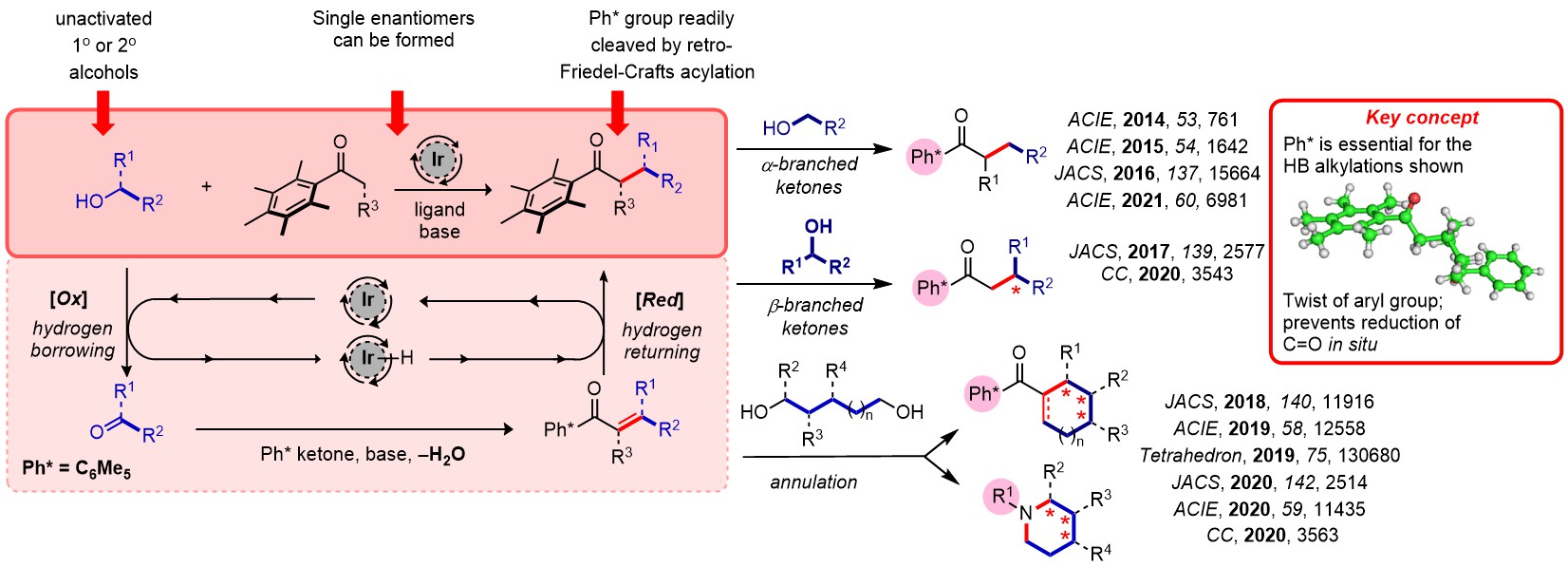
Hydrogen Borrowing
The use of reversible catalytic redox processes (termed hydrogen borrowing catalysis) is a powerful way of making C-C bonds. The reversible nature of the redox reactions mean that reactive intermediates can be generated, and then captured, in situ. We have developed the Ph* group to overcome a number of previous problems centered around a lack of reactivity of the carbonyl substrates and the conditions employed, and this has allowed us to make complex structures or to make single stereoisomers of the products. Our goal is to greatly enhance the range of products that can be formed via enolate chemistry.
Catalytic Dearomatisation
We are also interested in the role that dearomatising reactions can play in organic synthesis. Recently, we have developed dearomatising reactions that utilise catalytic amounts of rhodium and that are able to form new C-C bonds in the dearomatisation process. This exciting new area enables a link to be made between the areas of aromatic and non-aromatic chemistry and allows us to rapidly prepare complex 3-D templates from 2-D aromatic precursors.
Natural product synthesis
As we develop a new method, it is important to test it in the area of total synthesis, and we concentrate on finding the limits of our new methodology with application to complex and biologically active targets. A selection of recently completed targets is shown below, together with the original methodology project that enabled the synthesis.