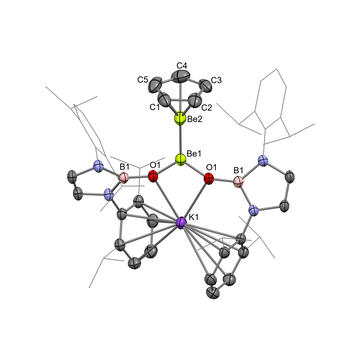
A complex with a highly polarized Be–Be bond.
Research led by Dr Josef Boronski and Professor Simon Aldridge FRS in the Department of Chemistry has led to the synthesis of a complex which behaves as a source of the nucleophilic beryllyl anion. This anion formally contains a beryllium(0) atom, which is a new oxidation state for this element. Notably, the beryllyl anion is also isoelectronic with the boryl anion, and this work highlights the hitherto unknown similarities between the homo-elemental bonding of beryllium and boron, which are “neighbours” in the Periodic Table. The study is published today in Nature Chemistry.
Building on previous work in which they generated the first example of a Be–Be bond, the team were able to substitute the ligands of diberyllocene and generate a strongly polarized Be–Be bond. Quantum chemical calculations and X-ray diffraction data revealed that the bond should be formulated as a mixed-oxidation state species with a Be0→BeII donor-acceptor-type interaction.
This oxidation state assignment was backed up by experiments showing their polarized Be–Be complex acting as a source of a nucleophilic beryllium(0)-containing beryllyl anion, leaving behind a beryllium(II) complex.
Dr Josef Boronski says:
Due to its toxicity, beryllium’s molecular chemistry has long been seriously underexplored. It’s fantastic to see that fundamental studies of this kind can still shed new light on areas of the Periodic Table that we think we know extremely well.
The properties of element number four – beryllium – are currently poorly understood, due in large part to its extreme toxicity. A comprehensive knowledge of beryllium’s chemistry is, however, critical for understanding fundamentals of chemical bonding and reactivity of the lightest elements. This work is amongst the first to reveal the ways that beryllium, ostensibly a metal, bridges the gap between s-block metals and p-block non-metals such as boron.
You can read more today in Nature Chemistry: A nucleophilic beryllyl complex via metathesis at [Be–Be]2+, and an accompanying research briefing from Dr Josef Boronski.