Researchers from Professor Luet Wong’s and Jeremy Robertson’s groups and their team at the Oxford Suzhou Centre for Advanced Research (OSCAR) have recently developed a new approach to selectively target C–H bond oxidation at a wide variety of positions in cyclic amines.
Using specially evolved cytochrome P450 enzymes the team achieved enantioselective functionalization of all unreactive C–H bonds in a collection of cyclic amines. These nitrogen-containing rings are an important precursor for many drug molecules, with around 60% of the United States' FDA (Food and Drug Administration) approved small-molecule drugs containing this kind of structural motif.
In the study, recently published in Nature Synthesis, the team used molecular dynamics simulations to iteratively predict how cyclic amines would dock into the enzyme. With just a few rounds of simulations and genetic modifications they were able to quickly block unwanted or non-productive docking “poses”, designing mutations that greatly increase both product selectivity and enzyme activity.
You can read more on the study in this short briefing, or in an interview with the researchers in Chemistry World.
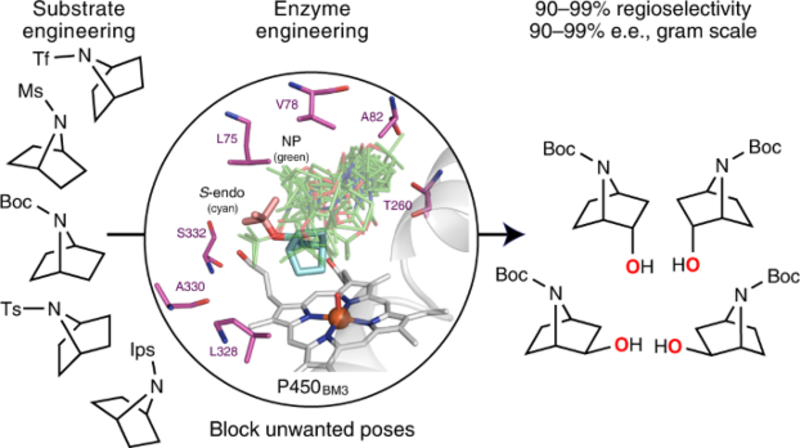
A schematic representation of the team’s method – enzyme mutations are engineered to suit various substrates, leading to highly selective oxidation of a variety of cyclic amine’s at different positions round the ring.